Cool Info About How To Draw Lewis Structure Diagrams

Determine the total number of valence electrons in a molecule.
How to draw lewis structure diagrams. Examples include nacl, mgf2, k2o, and al2o3.my website: How to draw bohr diagrams & lewis structures 8 p 8 n. This chemistry video explains how to draw the lewis structures of ionic compounds.
Science ap®︎/college chemistry molecular and ionic compound. Then, determine whether the atoms are held together by a single, double, or triple. Write the number of neutrons and the number of protons in the nucleus.
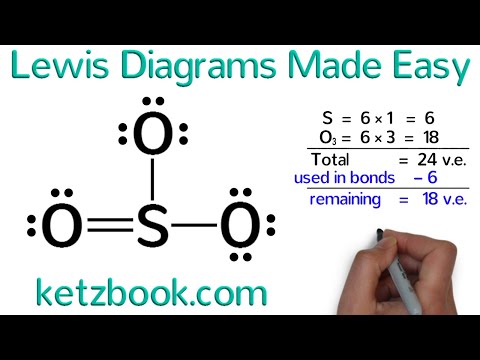
Check your understanding of lewis diagrams in this set of free practice questions designed for ap chemistry students. How to draw lewis diagrams. The steps to draw the lewis structures of various types of compounds are given below:
To learn more about this topic and other related topics, register with. Steps to draw lewis structure. 2) carbon is in the 4 th group, so it has 4 valence electrons.
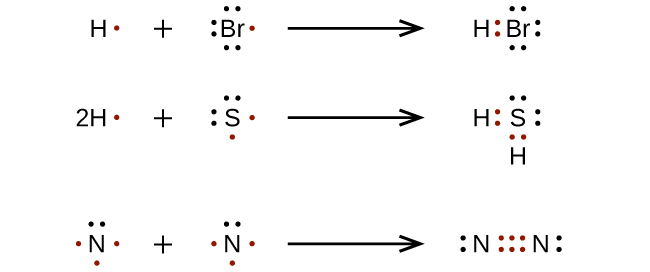
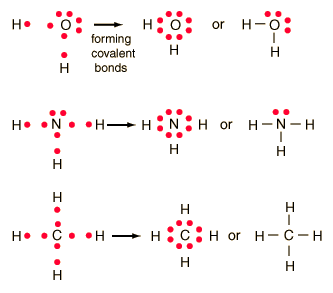
Determine the total number of valence electrons in a molecule. The lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom. Determine the total number of valence electrons in a molecule.
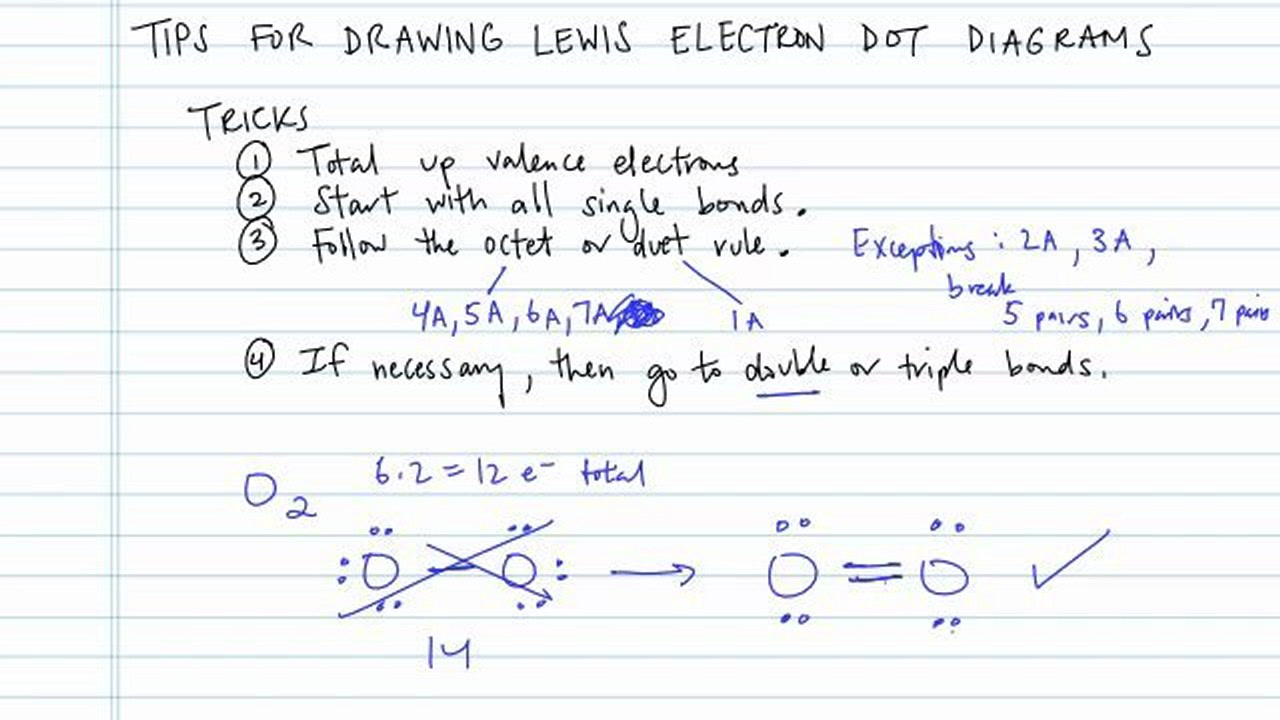
How to draw lewis diagrams. 3) the number of dots must equal the number of valence electrons. How to draw lewis structures 1.





/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)